Medicinal plants have been identified and used throughout human history. Ginseng is one of most commonly used medicinal herbs, and has been long used in China, Korea, and Japan as well as among North America Indians.
However, the therapeutical response of medicinal herbs including ginseng varies, is not satisfactory in most cases and could be even toxic if not used properly because traditional preparation of ginseng as well as other medicinal herbs has three inborn shortcomings:
- Quality: There are thousands of chemical ingredients in medicinal herbs, some are of therapeutical value, some are not and even toxic. Modernization of herbal drug development requires the enrichment of most potent ingredients to maximize the clinical efficacy, and adquate removal or minimization of toxic ingredients to reduce adverse and side effects. Most herbal drugs and natural products are powder or raw extract of medicial herbs, their chemical ingredients are not well characterized, and pharmacological efficacy is also not clearly addressed.
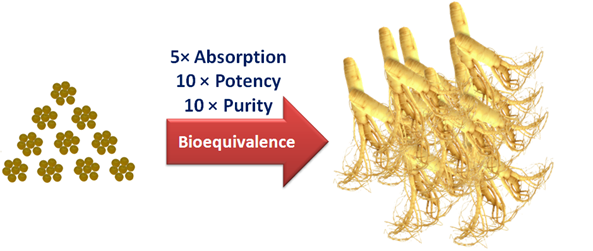
- Quantity:In oder to exert a noticeable clinical efficacy, a minimal therapeutical blood concentration of active ingredients must be reached. However, the amount of active ingredients in medicinal herbs is usually very low. Furthermore, active ingredients in tablet, capsule or powder preparations are poorly absorbed in the GI tract. Therefore, a large amount of raw herbs or herbal extract is required, which makes such treatments infeasible and unaffordable. For example, the minimal therapeutical concentration of dammarane sapogenin PPD, a most potent anti-cancer ingredient in American Ginseng, is 5ug/ml in blood. To reach this concentration, a daily oral dose is 250g for raw ginseng or ginseng powder, and 12g for purified ginsenosides, and the corresponding costs would be prohibitive.
III. Absorption / Bioavailability: Many active ingredients of medicinal herbs are saponins, flavonoids, etc., which are hydrophobic and poorly absorbed by GI tract in tranditional preparations such as tablets, capsules and powder. Modern research has shown that only 5% of PPD and PPT, the most potent ingredients in American and Asian ginsengs, respectively, enters blood after oral administration in traditional formulations. Therefore, the low absorption greatly compromises clinical efficacy of active ingredients of medicinal herbs.
In order to address the above problems, scientists from PLC spent tremendous efforts over 10 years and achieved two breakthroughs. The revolutionary PAMA and dripping pill (micropellet) technologies completely tackled the hindrances in quality, quantity and absorption.
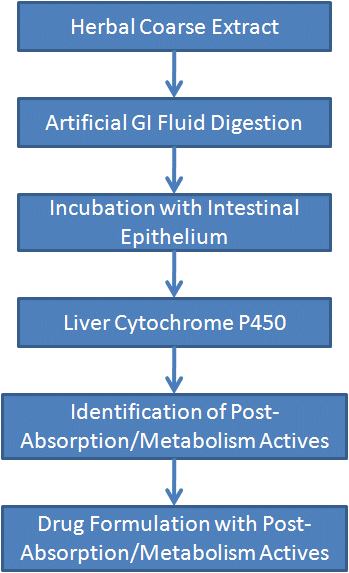
- Qualitative and Quantitative Analyses of Active Ingredients – Post-Absorption/Metabolism Actives Technology / PAMA
One main goal of herbal drug modernization is to characterize their active ingredients and pharmacological mechanisms, and traditional preparations are not able to accomplish it.
Before entering blood, the herbal ingredients are subjected to chemical hydrolysis in gastric acid, pancreatic juice and mucosal cells in the stomach and intestine, and then transformed by the cytochrome P450 system in the liver after entering the portal system, some ingredients are activated, and others are deactivated or secreted into the bile. After these processes, only few ingredients can be detected in blood, which makes the identification and characterization of active ingredients become possible.
Using the PAMA technology, we have successfully identified that the most active ingredients of ginseng are dammarane sapogenins [protopanaxadiol (PPD) and protopanaxatriol (PPT)], and their activities increase by 10-30 fold than their saponin precursors after the GI digestion/absorption and liver metabolism.
Furthermore, cell cultures and animal models were utilized to evaluate the effective dose of dammarane sapogenins, showing a minimal concentration of 1-5ug/ml is required for PPD or PPT to exert noticeable pharmacological effects.
- Absorption (Bioavailability) Enhancement Technologies – Solid Dispersion, Co-Solvency and Unsaturated Covalent Bonding
| In general, the drug particle size matters for drug absorption. In traditional preparations of herbal drugs (tablets, powder, and capsules), drug exists in a form of coarse particles, which greatly reduces their absorption (usually, less then 5%).
It is well-recognized that the higher the lipophilicity, the poor the oral absorption. Many active ingredients of herbal drugs such as dammarane sapogenins (PPD and PPT) are lipophilic, leading to a very low bioavailability in regular oral forms. After spending tremendous efforts over years, PLC’s scientists finally overcome the above barriers using following technologies: a. Solid Dispersion and Co-Solvency in Dripping Pills Greatly Increase Absorption PLC’s scientists creatively combined two technologies in a dripping pill (micropellet) drug form. Using proprietary solid dispersion techniques, dammarane sapogenins exist in molecular form (a solid solution or molecular level mixing) in dripping pills. Futhermore, the dripping pill adopts water miscible co-solvents as matrix, in which dammarane sapogenins have a high solubility. In addition, cosolvents (e.g., PEG, PVP and poloxamer) are miscible with water in all proportions. After their release at a molecular level with co-solvent dissolution, dammarane sapogenins form an aqueous solution with cosolvents in GI fluid, and are ready for rapid absorption by oral mucosa or GI tract. With these two breakthroughs, the absorption of dammarane sapogenins increases drastically, and a bioavailability of >40% has been achieved for the dripping pill preparation of PPD and PPT. b. Unsaturated Covalent Bonding Makes Powder, Tablet and Capsule Preparations Possible The cis double bonds present in unsaturated fatty acids cause kinks in their tails, thus interrupting its van der Waals interactions with neighboring fatty acids. This effect leads to an increased solubility and decreased melting points of unsaturated fatty acids. Inspired by this phenomenon, PLC’s chemists introduced double-bond into the tail structure of dammarane sapogenins, this unsaturated covalent bonding technology greatly increased the solubility of dammarane sapogenins by over 100 fold, without marked compromisation of their activities. This technology makes powder and capsule forms of dammarane sapogenins possible. With the solubility increase, the oral bioavailability of dammarane sapogenins in powder, tablet and capsule form increases to 25%~30%. |
  |
|

 Tiếng Việt
Tiếng Việt Indonesia
Indonesia Malaysia
Malaysia